 |
|
Biography
Yannick Wimmer was born in Steyr, Austria, in 1985. He studied physics at the Technische Universität Wien, where he received the degree of Diplomingenieur in 2012. He joined the Institute for Microelectronics in August 2012, where he is currently working on his doctoral degree. His current scientific interests include channel hot-carrier effects and NBTI.
Influence of Ring Size in Amorphous SiO2 on Hydrogen Kinetics
Hydrogen (H) is held responsible for many of the detrimental effects observed in the oxides of MOSFETs. It is suspected to be the main contributor to charged defects that can alter the electrostatic behavior of an entire device. Experimental observations and recent density functional theory (DFT) calculations have shown that atomistic hydrogen seems to move through the oxide material (here silicon dioxide (SiO2) ) by hopping from one bridging oxygen atom to another. Positive H (a proton) binds especially strongly to oxygen atoms.
The hopping process, wherein the proton jumps from one oxygen to another, is illustrated at the top of Fig. 1. When neutrally charged, the hydrogen atom can either become interstitial, break one of the bonds of the bridging oxygen or simply remain attached (if a feature nearby is capable of capturing the spare electron) (see the bottom of Fig. 1).
The probability that hopping will occur is determined by the energy barrier that has to be overcome for such a reaction to take place. Contrary to a crystalline structure, an amorphous SiO2 material can be subdivided into rings of many different sizes. It is of great interest if the H-hopping barriers show a dependence on ring size. Furthermore whether or not hopping rather occurs between neighboring oxygen atoms of the ring or preferably across the ring (cross-ring) (see Fig. 2) was investigated.
Calculations revealed that no correlation between behavior in the neutral state (see Fig 1.) and ring sizes could be deduced. It may seem odd at first glance that transitions into an interstitial state are also part of this investigation. It can be shown however that interstitial hydrogen cannot diffuse far before attaching again to one of the next oxygen atoms, which allows such binding in the neutral state. These reactions would therefore again resemble H-hopping, but now not every oxygen atom can participate.
The main conclusion to be drawn from our calculations is that cross-ring hopping has, on average, lower barriers than neighboring hopping, a finding that was previously reported for positive proton hopping. It can be shown that this also seems to hold true for the neutral charge state. We can therefore conclude that, independent of the charge state, cross-ring hopping (see Fig. 2) seems favorable.
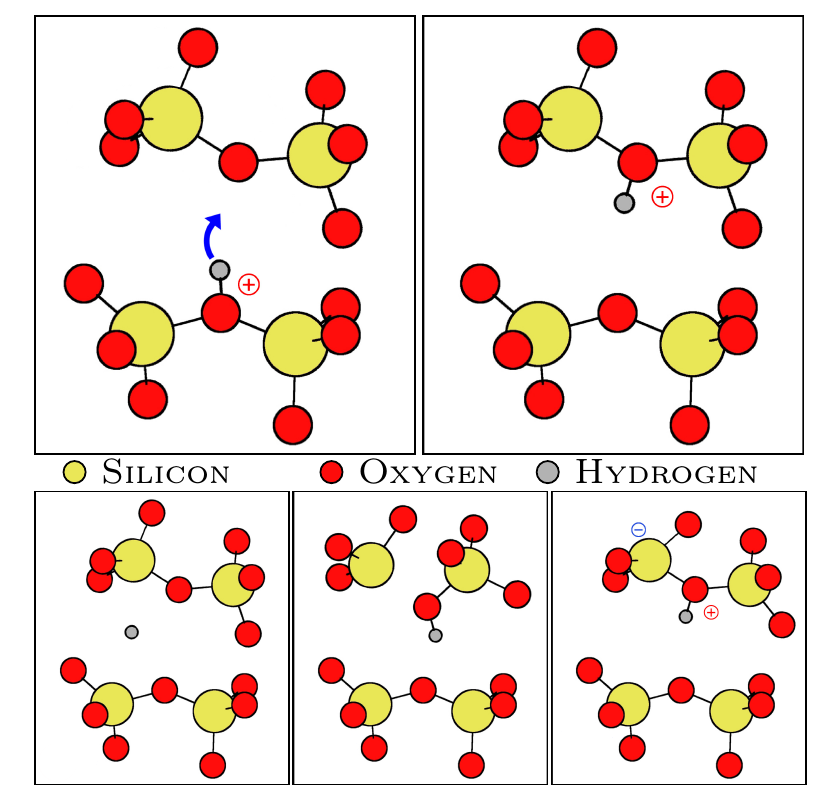
Fig. 1: Top: Hydrogen hopping transition in the positive charge state.
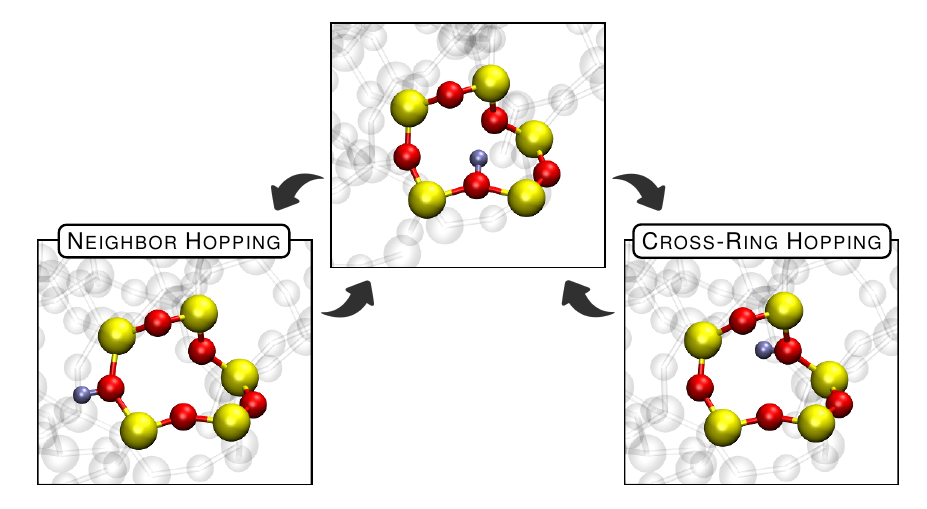
Fig. 2: A ten-member ring in SiO2 in which the positively charged hydrogen (proton) undergoes either a neighboring hopping or a cross-ring hopping transition.



