



Next: 9.3 Modeling of the
Up: 9. Intrinsic Stress Effects
Previous: 9.1 Cantilever Deflection Problem
9.2 Sources of Intrinsic Stress
In the first phase of the SiGe deposition process, islands
with varying crystal orientation are formed and grow isotropically. These individual islands which first form on a substrate usually exhibit compressive stress [136].
In the course of further deposition these islands start to coalescence, which forces the islands to grow in the height instead of in a direction parallel to the substrate surface.
The islands are subsequently transformed from an island shape to a grain-like shape.
The orientation of the crystal structure in a single grain (e.g. perpendicular to the substrate surface) is independent of the neighboring grains, since due to the amorphous substrate, it is not possible to evolve a perfect crystal structure in the first atom layers [137].
For the stress aspect the deposition process plays a key role. At first it should be noted that the deposition takes place at elevated temperatures. When the temperature is decreased, the volumes of the grains shrink and the stresses in the material increase. Furthermore, the stress gradient and the average stress in the SiGe film depend on the Si-Ge ratio which can be controlled by the silane (SiH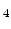 ) and germane (GeH
) and germane (GeH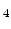 ) flow, the substrate temperature, and the deposition technique which is usually LPCVD (low pressure chemical vapor deposition) or PECVD (plasma enhanced chemical vapor deposition). It was observed that the average stress becomes more compressive, if the Ge concentration decreases [138]. Thus it is expected that a film with higher Ge concentration has a higher degree of crystallinity and larger grains, which leads to higher film density and to higher tensile stress.
) flow, the substrate temperature, and the deposition technique which is usually LPCVD (low pressure chemical vapor deposition) or PECVD (plasma enhanced chemical vapor deposition). It was observed that the average stress becomes more compressive, if the Ge concentration decreases [138]. Thus it is expected that a film with higher Ge concentration has a higher degree of crystallinity and larger grains, which leads to higher film density and to higher tensile stress.
The intrinsic stress observed in thin films has generally the following main sources [133]:
- Coalescence of Grain Boundaries:
In the early stage of the film growth the individual grain islands grow, until they make contact to adjacent islands (see Fig. 9.5a). The isolated islands have a relatively high surface energy  compared to the relatively low energy
compared to the relatively low energy  between the island interfaces. Therefore, the net free energy in the system can be reduced by replacing the surfaces by interfaces. If the gaps between the islands are small enough, cohesion begins to develop between the islands, and the system can lower its net free energy by closing up these gaps as depicted in Fig. 9.5b. In the course of zipping up the interfaces, the participating islands become elastically strained and a tensile stress is generated [139].
between the island interfaces. Therefore, the net free energy in the system can be reduced by replacing the surfaces by interfaces. If the gaps between the islands are small enough, cohesion begins to develop between the islands, and the system can lower its net free energy by closing up these gaps as depicted in Fig. 9.5b. In the course of zipping up the interfaces, the participating islands become elastically strained and a tensile stress is generated [139].
Figure 9.5:
Principle of coalescence. Structure of the grain island before a) and after b) coalescence.
a) ![\includegraphics[width=0.35\linewidth]{fig/coalescence1}](img960.png)
b) ![\includegraphics[width=0.38\linewidth]{fig/coalescene2}](img961.png)
|
- Misfit Stress:
The lattice constants for the thin film  and the substrate
and the substrate  are generally different (see Fig. 9.6a). Because of the deposition process the crystal lattice of the thin film and the substrate are forced to line up perfectly at the interface and stress arises as shown in Fig. 9.6b. The influence of these misfit stresses is only significant in the initial phase of thin film deposition [140], because of the local lattice adaption at the interface area. Furthermore, misfit stress can arise between the grain boundaries because of a different crystal orientation of neighboring grains.
are generally different (see Fig. 9.6a). Because of the deposition process the crystal lattice of the thin film and the substrate are forced to line up perfectly at the interface and stress arises as shown in Fig. 9.6b. The influence of these misfit stresses is only significant in the initial phase of thin film deposition [140], because of the local lattice adaption at the interface area. Furthermore, misfit stress can arise between the grain boundaries because of a different crystal orientation of neighboring grains.
Figure 9.6:
Different lattice constants a) leads to misfit stress in the film b).
|
|
- Annealing of the Film:
An annealing step after deposition of metal films produces a better crystalline arrangement and an increase of the material density, which results in a shrinkage of the film [141]. As long as the film is attached to the substrate the film is prevented to shrink and a tensile stress is developed.
- Grain Growth:
Due to the elimination of grain boundaries a minimum in the total energy of the system can be reached. So grain growth means that the volumes of the individual grains become larger and the number of grains and its boundaries decrease. The grain growth stops at this minimum energy. Since grain boundaries are less dense than the grain lattice [142], the elimination of grain boundaries leads to a densification of the film and, therefore, to a build up of tensile stress.
- Annihilation of Excess Vacancies:
The annihilation and the dynamics of the crystal vacancies produce a local volume change which leads to stresses in the film when it is attached to the substrate.
The vacancies annihilate in the grains, at the grain boundaries, at the free surface of the film, and at the surface of the internal cavities.
If vacancies are annihilated at the free surface and at internal cavities, no
stress is produced.
When vacancies annihilate at a grain boundary, there is a gap.
In addition vacancy annihilation in the grains leads to removal of atoms from the grain boundaries to the interior of the grains, which also leads to a gap. Both cases
cause a motion of the crystals towards each other in order to close the gap. This would produce a planar contraction of the film, if it is not attached to the substrate.
But since the substrate prevents contraction, a tensile stress is built up instead [133].
- Thermal Stress:
This stress is caused by the different thermal expansion coefficients of the thin film and the substrate in case of a temperature change after deposition and the fact that at least a part of the film's base area is attached with the substrate. Therefore, thermal stress develops during cooling down to room temperature.
- Insertion of Excess Atoms:
It is assumend that the film growth process can add atoms to the film in two ways [143]. Most of the material is added on the top surface by traditional crystal growth mechanisms, where each layer of atoms is deposited onto the underlying crystalline lattice. The second mechanism is the incorporation of excess atoms into the grain boundaries, which creates a compressive stress in the film [144].




Next: 9.3 Modeling of the
Up: 9. Intrinsic Stress Effects
Previous: 9.1 Cantilever Deflection Problem
Ch. Hollauer: Modeling of Thermal Oxidation and Stress Effects
![]() ) and germane (GeH
) and germane (GeH![]() ) flow, the substrate temperature, and the deposition technique which is usually LPCVD (low pressure chemical vapor deposition) or PECVD (plasma enhanced chemical vapor deposition). It was observed that the average stress becomes more compressive, if the Ge concentration decreases [138]. Thus it is expected that a film with higher Ge concentration has a higher degree of crystallinity and larger grains, which leads to higher film density and to higher tensile stress.
) flow, the substrate temperature, and the deposition technique which is usually LPCVD (low pressure chemical vapor deposition) or PECVD (plasma enhanced chemical vapor deposition). It was observed that the average stress becomes more compressive, if the Ge concentration decreases [138]. Thus it is expected that a film with higher Ge concentration has a higher degree of crystallinity and larger grains, which leads to higher film density and to higher tensile stress.